Current cancer treatments like chemotherapy often have severe side effects because they affect not only cancer cells but also healthy cells. This is the reason why several research undertakings have focused on finding and developing novel and alternative treatment options that are safer and better. These range from immunotherapy to advanced targeted therapies like light-induced gene therapy. Bacterial delivery therapies are another example of target therapy.
Scientists from the University of Massachusetts Amherst and Ernest Pharmaceuticals have been developing and perfecting a specific bacterial therapy called BacID that uses genetically engineered Salmonella to deliver cancer-fighting drugs directly into tumors. This innovation holds promise for treating high-mortality cancers such as liver, ovarian, and metastatic breast cancer with enhanced safety and efficacy compared to conventional therapies.
Targeted Therapy Using Bacterial Delivery System: How a Genetically Engineered Salmonella is Used to Deliver Drugs Directly to Cancer Cells
Background
Effective cancer treatments require precision targeting of cancer cells to ensure both effective delivery and better internalization of therapeutic agents. Several targeted delivery systems using bacteria have been explored. However, despite promises, most of these bacterial therapies faced challenges with poor tumor colonization and limited therapeutic efficacy.
Nevertheless, to address the aforementioned problems, scientists have genetically engineered an intracellular-delivering or ID strain of Salmonella by manipulating the expression of the flhDC gene. This gene is responsible for regulating flagella production and cell invasion. This control is key to enhancing bacterial colonization and therapeutic delivery.
This bioengineered Salmonella is an iteration. It represents the culmination of more than a decade of research by Vishnu Raman, co-founder and chief scientific officer of Ernest Pharmaceuticals, and Nele Van Dessel, a bioengineer who developed the bacterial delivery system as a post-doctoral researcher in the Forbes Lab of the University of Massachusetts Amherst.
Clinical trials with participating patients are set to begin in 2027. “This is exciting because we now have all the critical pieces for getting an effective bacterial treatment for cancer,” said Neil Forbes, senior author of the research published in late 2024 in the journal Molecular Therapy and professor of chemical engineering at the University of Massachusetts Amherst.
Mechanism and Findings
Scientists essentially developed a system to control when the bacteria invade cancer cells and release the drug. This is crucial for maximizing effectiveness and minimizing side effects.
“One core part of this technology is the controlled activation of flagella,” Raman explains. “And the other core part is once the bacteria go inside cancer cells, we engineered them with a suicide circuit. So they rupture on their own and deliver the therapy inside the cancer cell.”
The process works via intravenous injection of engineered Salmonella. These then accumulate in tumors. The immune system clears the bacteria in healthy tissues within two days.
Bacteria in healthy tissues are cleared by the immune system within 2 days. Aspirin is administered 3 days after the injection. This triggers the bacteria to invade cancer cells and release the drug. The bacteria then self-destruct after releasing the drug. The following are further details:
• Controlling the Flagella Production: The flagella of Salmonella is essential to cellular invasion. Controlling its production via the flhDC gene is key to enhancing bacterial colonization and therapeutic delivery.
• The Aspirin-Responsive Promoter: A novel aspirin-responsive promoter was used to control flhDC expression. Tags like ssrA degradation were also used to fine-tune protein stability. These modifications allowed precise control over bacterial invasion and dispersal in tumors to overcome the limitations in previous studies.
• Quality of the Engineered Salmonella: The researchers have also made the bacteria much safer than previous attempts to reduce the risk of infection. It also self-destructs once inside the cancer cells to release the therapeutic drug.
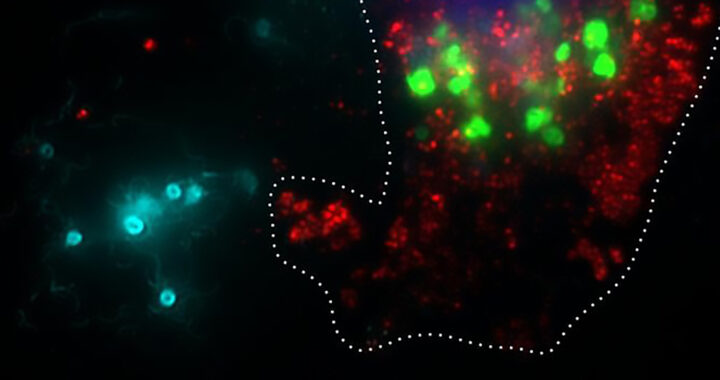
• General Findings From Experiments: Controlling flhDC significantly increased tumor colonization in a mouse model. Inducing flhDC promoted bacterial escape from intracellular vacuoles to enhance the delivery of therapeutic proteins. Deleting the sseJ gene further improved protein release by preventing vacuole escape.
• Efficiency and Potential for Scaling: The multiplication of the engineered Salmonella inside tumors enabled the delivery of significantly higher drug concentrations. This makes therapy both more potent and cost-effective.
Implications
The scientists presented a groundbreaking approach to bacterial therapy in oncology that used bioengineered Salmonella to deliver therapeutic proteins directly to cancer cells.
It fundamentally represents the combination of precision, safety, and convenience. To be specific leveraging the natural tumor-targeting abilities of bacteria minimizes harmful side effects while maximizing therapeutic impact.
Note that the integration of simple and patient-friendly phases, such as outpatient procedures and taking aspirin, further enhances it as a revolutionary cancer treatment method.
The potential application for treating aggressive and late-stage cancers is also promising. This comes from tumor-seeking capabilities to target difficult-to-access tumors, accumulate in cancer cells, and deliver higher doses of therapeutic drugs.
Cancer cells in advanced stages also often develop resistance to conventional drugs. The therapy can be useful in delivering novel drugs like therapeutic proteins.
Nevertheless, based on the experiments involving mouse models, the team is expecting to enter clinical trial by 2027. More observations are needed to determine how observations from animal models translate to humans.
FURTHER READINGS AND REFERENCES
- Raman, V., Hall, C. L., Wetherby, V. E., Witney, S. A., Van Dessel, N., and Forbes, N. S. 2024. “Controlling Intracellular Protein Delivery, Tumor Colonization and Tissue Distribution Using the Master Regulator flhDC in a Clinically Relevant ΔsseJ Salmonella strain. In Molecular Therapy. Elsevier BV. DOI: 1016/j.ymthe.2024.12.038